Bcs Class 2 Drug
ABOUT AUTHOR:AnilkumarDr.Ambedkar Institute of TechnologyBangalore, Indiaanilkumar3092@gmail.comINTRODUCTIONThe dissolution rate of drug from tablet is affected by its active ingredient’s surface area and consequently, affects in oral bioavailability of the product. The development of formulations containing poorly-water-soluble drugs for oral delivery can be achieved by improving their dissolution. It has been found that increasing the available surface area by reducing the particle size can often markedly improve dissolution rates and lead to dramatic improvements in bioavailability.
In some cases, the decreasing drug particle through micronized powder by milling tends to agglomerate or accelerate the polymorphic conversion. According to the differences of solubility and dissolution rates of polymorphs, the bioavailability of pharmaceuticals depends on polymorphous crystals. It has been shown that the polymorph in amorphous form of drug usually dissolves more rapidly than the corresponding crystalline form.
Therefore the dissolution and bioavailability of formulation containing active ingredient in amorphous form including pseudopolymorphs form such as solvates would be increased. On the other hand, the processes in making tablets, including blending, granulating, drying and especially compressing affected therapeutic property of the drug because polymorphic forms, crystal habit, size and surface area would be changed during these processes. REFERENCE ID: PHARMATUTOR-ART-2019A new technique of tablet preparation was patented, a chargeable pharmaceutical tablet which could solve the mentioned problems. The tablet was prepared by loading a blank tablet with liquid form of the active pharmaceutical ingredient. In this study, candesartan displays a poorly-water-soluble drug, which results in low and erratic oral bioavailability.
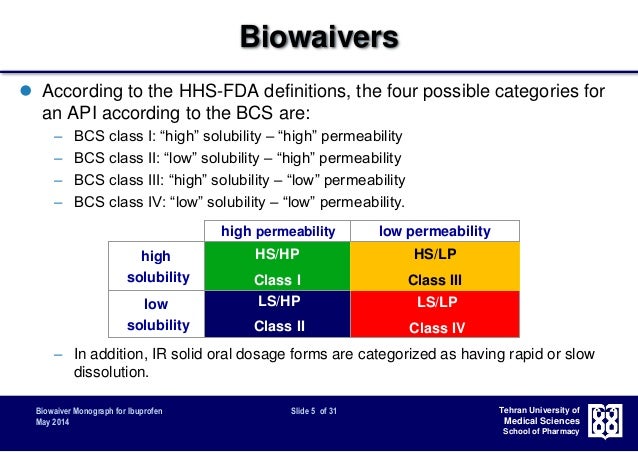
The Biopharmaceutics Classification System (BCS) Guidance. FDA has issued a final guidance entitled Waiver of In-vivo Bioavailability and Bioequivalence Studies for Immediate Release Solid Oral.
Attempts were made to enhance the dissolution of candesartan using “a drug-solution-dropping” technique which had success for water-soluble drug, chlorpheniramine maleate. In vitro drug dissolution rate was used as main criteria for comparison between both kinds of blank tablet with dropping drug solution and also with conventional tablet prepared by DC method. 2Therapeutic effectiveness of a drug depends upon the bioavailability and ultimately upon the solubility of drug molecules. Solubility is one of the important parameter to achieve desired concentration of drug in systemic circulation for pharmacological response to be shown. Currently only 8% of new drug candidates have both high solubility and permeability.
3 The solubility of a solute is the maximum quantity of solute that can dissolve in a certain quantity of solvent or quantity of solution at a specified temperature. In the other words the solubility can also define as the ability of one substance to form a solution with another substance. The substance to be dissolved is called as solute and the dissolving fluid in which the solute dissolve is called as solvent, which together form a solution.
The process of dissolving solute into solvent is called as solution or hydration if the solvent is water. 4 The transfer of molecules or ions from a solid state into solution is known as dissolution. In essence, when a drug dissolves, solid particles separate and mix molecule by molecule with the liquid and appear to become part of that liquid. Therefore, drug dissolution is the process by which drug molecules are liberated from a solid phase and enter into a solution phase. The use of poorly soluble drugs has a number of drawbacks such as increasing the dosage, administration frequency and the resultant occurrence of side effects. Furthermore, the rate-limiting step in the absorption process for poorly water-soluble drugs is the dissolution rate of such drugs in the gastro intestinal fluids rather than the rapidity of their diffusion across the gut wall; it is however, important to improve the oral bioavailability of poorly water soluble drugs by improving their dissolution rate and solubility.
Ilwis 3.3 with crack. ILWIS 3.3 Download page. ILWIS 3.31 Update. ILWIS 3.31 is a service pack on ILWIS 3.3. Some new functionality has been added to the Hydrologic Flow Operations and the Spatial Multiple Criteria Evaluation (SMCE); some bugs were fixed.
TECHNIQUES OF SOLUBILITY ENHANCEMENTThere are various techniques available to improve the solubility of poorly soluble drugs.
The Food and Drug Administration (FDA or Agency) is announcing the availability of a guidance for industry entitled “Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System.” This guidance finalizes recommendations for sponsors of investigational new drug applications (INDs), and applicants who submit new drug applications (NDAs), abbreviated new drug applications (ANDAs), and supplements to these applications for immediate-release (IR) solid oral dosage forms, and who wish to request a waiver of an in vivo bioavailability (BA) and/or bioequivalence (BE) study requirement.
AIRCRACK DRIVERS FOR MAC - It is essentially a dictionary attack. Because at one time, i used airodump-ng with mon mode enabled and the other time with mon mode disabled I used airodump-ng -c 1 -w 'data file name' -bssid If you want me to help you, you need to improve your basic computer skills and be MUCH more patient. You make an excellent. Install AirCrack-ng on Mac OSX 10.8 Mountain Lion easily. Installing Xcode, Xcode Command Line tools and Macports is all that's required for AirCrack-ng to work on the latest OS X. Rausb0 aircrack for mac download.
Submit Comments
You can submit online or written comments on any guidance at any time (see 21 CFR 10.115(g)(5))
If unable to submit comments online, please mail written comments to:
Dockets Management
Food and Drug Administration
5630 Fishers Lane, Rm 1061
Rockville, MD 20852
All written comments should be identified with this document's docket number: FDA-2015-D-1245.
Regulated Product(s)
Topic(s)